BiXAb®
Technology
Science
Introduction
BiXAb® AT THE CORE OF BIOMUNEX’s INNOVATION
Being based on an IgG format, BiXAb® antibodies are endowed with natural drug-like properties and have excellent biophysical and manufacturing properties.
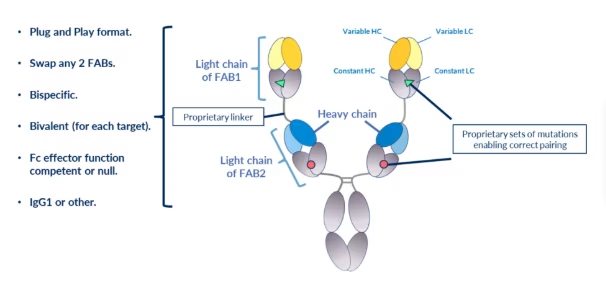
BiXAb® Features
(01)
Speed
The modular “Plug and Play” system employed for the construction of BiXAb antibodies leads to extremely short generation times. From Fab sequence identification made in silico to BiXAb purification and characterization is typically less than 6 weeks.
(02)
Excellent Pairing
To ensure correct pairing of the two Light chains, a series of proprietary mutations (pairs) have been designed (described and covered by two patent families), in the constant regions of the Fabs that can be used in various combinations to insure correct light chain:heavy chain pairing.
(03)
Linkers
A series of proprietary linkers, based on natural human immunoglobulin sequences, have been designed (described and covered by two patent families), that have sufficient length and flexible rigidity to permit efficient binding of the internal Fab (verified experimentally in many experiments; see article published in Frontiers in Immunology by Rabia et al 2023). These linkers also contribute significantly to the many advantageous biophysical and functional properties of these bi-specific antibodies.
(04)
Valency
The BiXAb technology has been initially developed as a bivalent (per target) and bi-specific antibody platform possessing tetra-valency overall. This permits the BiXAb antibodies to bind targets with affinities similar to native monoclonal antibodies and profits from the advantages of “avidity driven binding” seen with normal Mabs. This can be particularly important when one of the targets has poor expression.
(05)
Excellent Drug-like Properties
The excellent drug-like properties of the BiXAb antibodies are due to the fact that the format is based on a native IgG structure with the internal Fab linked to a second correctly assembled Fab attached by a natural, immunoglobulin-derived linker sequence. These properties include excellent binding affinities, lack of aggregation, low risk of immunogenicity and high thermal stability.
(06)
Robust Manufacturability
Due to the robust biochemical and biophysical features of the “naturally folded” BiXAb structure, excellent manufacturability in CHO cells is achieved with the BiXAb format. This can have a major impact on CMC efficiency, cost of goods and clinical supply.
(07)
Strong IP Protection
BIOMUNEX has developed a very strong IP protection position and is continuously solidifying patent protection of its BiXAb technology as well as the innovations and improvements that continue to differentiate the BiXAb technology from most of those used in the bsAb field.
Bi- and multi-specific Bixab® antibodies developed by Biomunex
BIOMUNEX discovers and develops new treatments based on disruptive data driven biological approaches in oncology for patients with unmet medical needs, using its bi- and multi-specific antibody technology BiXAb®.
Overall, all BiXAb® programs have demonstrated the superior added value of the BiXAb® technology compared to traditional multi-specific antibodies formats : rapid production, quick generation of the IP covering the molecule, excellent drug-like properties, manufacturability and low COGS (Cost of Goods Sold), and finally versatility and multi-specific capabilities.
Several patent families have been filed to protect such disruptive approaches.